You’ve most likely heard of people which needed part in a number of studies with time, some might have found a effective method to their existence threatening disease, others may possibly not have been so lucky. This may cause you to wondering the reasons studies are important and the best way to make any difference in this kind of profession ongoing to move forward.
The first reason numerous studies are important is they identify how new potential treatments work. They’re presented to particular individuals who’ve selected to join up, this sort of person then monitored regularly to understand the way a treatment solutions will work, whether it creating a difference and exactly how they respond to the brand-new medication.
Another excuse why this testing phase is really imperative before any drugs are released for that public may be the developers desire to make certain it’s safe. What this means is to be able to they might really identify the actual way it will customize the is allow it to humans, individuals with and without serious illnesses, to ensure that it’ll as it is designed to and can produce a positive improvement in several people’s lives later on.
With the medical study, some participants is going to be provided one dose with other people receiving another. This is often familiar with uncover the most effective suggested dosage to supply while using the medication once released. The goal is so that the dosage directions are safe and effective.

Further, you will find that numerous studies give researchers and developers the opportunity to uncover the final results from the medication or device. Numerous studies can be used medications, medical equipment much more which should help these medicinal companies obtain product available and distributed across the marketplace.
You have to understand that numerous studies is only a small area of the research process and just a number of these products really achieve everybody, there are numerous projects which are not approved and thus never acquire a hospital or Pharma Franchise Company shelf.
The procedure for developing a new medication or strategy is a extended and daunting one and needs a extended time. It comes down lower lower to the thought of progression of the product, this is done within the laboratory setting. Out of this level the medication or strategy is purified and tested within the laboratory. Right now most commonly it is test tube testing, that may require six a lengthy time for you to master. This will make it usually tested on animal test subjects to make sure its safety before even reaching human trials.
Individuals medications reaching human testing acquiring the best possibility of success. The trial isn’t signed off unless of course obviously clearly all of the research right before that time are able to see the safety factor is not compromised. Should you understand that numerous studies cannot guarantee your safety and medications have a danger, it might be you to definitely certainly certainly consider the chance benefits along with the risks to find out when the benefits over-shadow the danger and can produce a positive impact on your existence, prior to deciding to consider involved with any study such as this.
You have to understand that even if you choose to be a part of a medical trial project, there’s no make certain the item makes it for that public. Very number of of people products really achieve the general public which isn’t only a cheap exercise either. It requires many years of dedication and keenness to get a new medication for the shelf and distributed around individuals who are able to be helped because of it.
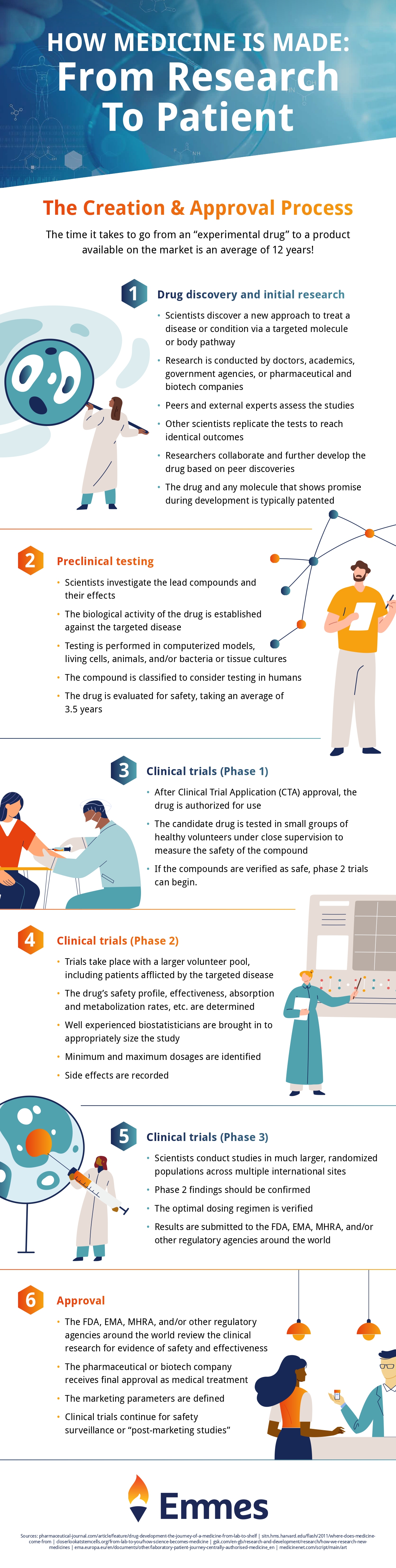
Check out the infographic below to see how your medicine is made, from start to finish!
Infographic provided by The Emmes Company, a clinical data management organization